
• Research Highlight
Symptoms of depression can be difficult to manage, especially for people with treatment-resistant depression, a persistent and severe form of the disorder. Fortunately, new therapies are emerging for such difficult-to-treat conditions. One of them is the drug ketamine, which numerous NIMH-funded studies have shown has real, lasting effects on people with mood disorders such as depression.

The discovery of ketamine has been a game-changer for people with severe depression, who often need quick relief from life-threatening symptoms. While most antidepressants take weeks or months to take effect, ketamine works within hours to significantly reduce depression symptoms in people for whom other treatments have not worked.
Despite ketamine’s effectiveness as an antidepressant, there are serious concerns that limit its use, including problematic side effects and a high risk of misuse. To address these concerns, the National Institutes of Health (NIH) is investing in finding drugs that take advantage of the therapeutic effects of ketamine while avoiding the negative ones.
What did the researchers observe in the study?
New research funded through the NIH Blueprint Neurotherapeutics Network Program for Small Molecules examined a new ketamine-related drug known as RR-HNK. RR-HNK is a metabolite or byproduct, of ketamine that remains when the body breaks it down. RR-HNK showed antidepressant effects in preclinical animal studies but it had not yet been tested in humans.
The study involved extensive collaboration of researchers from the intramural programs of the National Institute of Mental Health, the National Center for Advancing Translational Sciences, and the NIH’s National Institute on Aging; Duke University and the University of Maryland School of Medicine; and other national and international institutions.
What did the researchers do in the study?
This study examined the safety, tolerability, pharmacokinetics (how the drug moves through the body), and pharmacodynamics (how the drug affects the body) of RR-HNK for the first time in humans.
Participants were healthy adults between 18 and 65 years old. A total of 74 people participated in three randomized trials: 55 received RR-HNK and 19 received an inactive placebo. Both the drug and the placebo were administered intravenously, and the participants and researchers were blinded to which group the participants were in.
- In trial 1, participants received one of six dose levels of RR-HNK only once.
- In trial 2, participants received one of two dose levels of RR-HNK four times for 2 weeks.
- In trial 3, participants received a single dose of RR-HNK and their cerebrospinal fluid (a fluid that surrounds the brain and spinal cord) was collected.
The main goal of the study was to determine if the drug is safe by first testing it in healthy adults without a diagnosed mental health condition. Throughout the study, researchers closely monitored adverse events, such as negative side effects. The comprehensive safety profile included physical examinations; laboratory results; vital signs; electrocardiograms of cardiac activity; and ratings of mood, suicide risk, and dissociation and sedation symptoms. Additionally, participants were asked to inform study staff if they experienced any concerns or side effects at any time.
The researchers also collected blood and urine samples from all participants before, during and after receiving the medication. These samples and the cerebrospinal fluid collected in trial 3 were used to test whether the drug entered the body and brain.
As a final exploratory step, the researchers used brain imaging to examine the participants’ gamma oscillations (a type of brain wave) before and after medication. This measure of the brain’s response to stimuli is one of the few available biomarkers of a drug’s effects on the brain.
What did the study find?
RR-HNK proved to be exceptionally safe, causing no serious adverse events and only mild side effects that resolved quickly without attention. Participants also reported no symptoms of sedation or dissociation. The positive safety profile was maintained at all doses tested and after multiple doses. Taken together, the results indicate that RR-HNK is safe and tolerable, with limited potential for abuse or misuse.
Cerebrospinal fluid confirmed that RR-HNK entered the brain and remained at detectable levels several hours after administration. The results also showed a response proportional to the dose of the drug, meaning that at higher levels of RR-HNK, the amount of the substance in the body also increased at the same rate. A predictable relationship between the amount of RR-HNK given and the amount of RR-HNK in the bloodstream is important to the clinical effectiveness of the drug, allowing doctors and researchers to precisely calibrate doses based on a person’s specific level and type of symptoms.
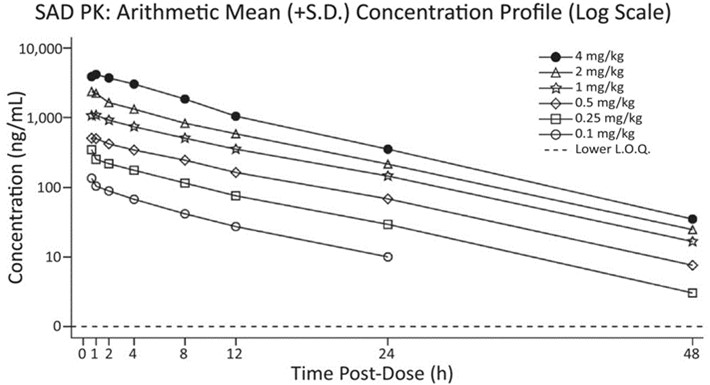
In the brain activity test, some participants who received low to moderate doses of RR-HNK, but not those receiving high doses or the placebo, showed an increase in the power of gamma oscillations. Preliminary evidence that RR-HNK produces a change in brain activity, strengthening the argument for its use as an antidepressant and providing a clinical biomarker to measure whether it works in future research. However, there was great variability in the results and, given the small number of participants, the researchers caution against drawing firm conclusions from these findings.
What do the results mean?
This study provides critical information on the safety, tolerability, and effects of RR-HNK in a diverse population of healthy adults. The findings from this early-stage study demonstrate that the ketamine metabolite does not cause the negative side effects of ketamine and is safe for use in humans. The results also help establish dosing parameters for use in future research and treatments.
These data, particularly a strong safety profile, support progress toward the next phase of research aimed at developing new therapies for people with difficult-to-treat mental disorders. Despite the small size of each trial, which makes some of the results difficult to interpret, the findings are promising for the future of mental health treatment. This study is a critical first step in NIMH’s mission to improve the treatment of mental illness through research, laying the groundwork for clinical trials to test whether RR-HNK effectively treats depression and other disorders.
Reference
Raja, S.M., Guptill, J.T., Mack, M., Peterson, M., Byard, S., Twieg, R., Jordan, L., Rich, N., Castledine, R., Bourne, S., Wilmshurst, M., Oxendine, S., Avula, SGC, Zuleta, H., Quigley, P., Lawson, S., McQuaker, SJ, Ahmadkhaniha, R., Appelbaum, LG… and Thomas, CJ (2024). A phase 1 evaluation of the safety, tolerability, pharmacokinetics and pharmacodynamics of (2R,6R)-hydroxynorketamine in healthy volunteers. Clinical and therapeutic pharmacology, 116(5), 1314-1324. https://www.doi.org/10.1002/cpt.3391
Subsidies
R01MH107615 , ZIAMH002857 , ZIATR000042 , ZIAAG000297







