
For patients with serious illnesses, timely access to effective medications is paramount. The European Medicines Agency (EMA) was created in part to help speed up drug approvals and ensure that these products are safe and effective. As stated in an article by Grünwald and Stargardt (2024):
The EMA [European Medicines Agency] was founded in 1995 primarily to harmonize the marketing authorization of pharmaceutical products in the EU and EEA… as there were substantial differences between European countries in terms of delay in launch and availability of pharmaceutical products.
The EMA had 3 key community procedures granting access to the markets of some or all EU member countries simultaneously.
- Centralized procedure (CP). If the EMA evaluates a medicine and grants it marketing authorization, this determination is binding on all member states of the European Union. CP was introduced in 1995 and was originally used only for “biotechnological processes, such as monoclonal antibodies, controlled gene expression or recombinant DNA technology.” The list of treatments evaluated under the PP has been expanded to include orphan drugs and substances against cancer, diabetes and HIV/AIDS (in 2005), viral diseases and autoimmune diseases/dysfunctions (in 2008) and therapeutic drugs advanced (e.g., cell and gene therapy) also in 2008.
- Mutual Recognition Procedure (MRP). In this case, the evaluation is carried out by a reference Member State, which the applicant can freely choose and whose decision is subsequently taken by all other Member States in which the applicant seeks market access. This procedure was adopted in 2001 and includes new treatments that are outside the CP, such as other pharmaceuticals and generics.
- Decentralized procedure (DCP). Adopted in 2005, this would allow pharmaceutical manufacturers to seek approval on a country-by-country basis. This is only eligible for new substances that are not governed by CP or MRP.
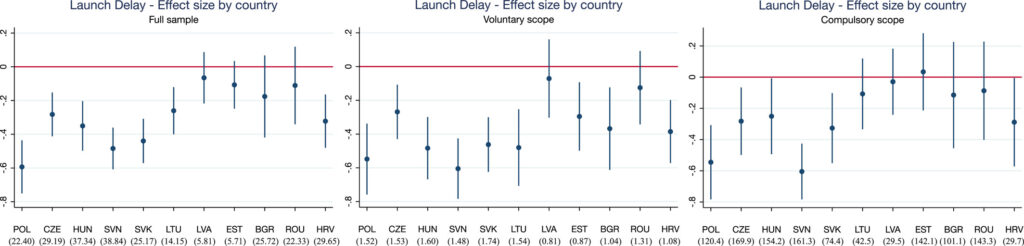
To examine the impact of these procedures, Grünwald and Stargardt (2024) perform a difference-in-difference analysis comparing the countries subject to these community procedures with those that were not. Specifically, with the enlargement of the EU, in 2004 the Czech Republic, Estonia, Hungary, Latvia, Lithuania, Poland, Slovakia and Slovenia (Cyprus and Malta also joined the EU on this date, but the authors did not have data on these countries). In 2007, Bulgaria and Romania joined the EU and then Croatia in 2013. By contrast, Belarus, Bosnia and Herzegovina, Kazakhstan, Russia, Serbia, Switzerland and Turkey never joined the EU. Using IQVIA sales data from 33 European countries, the authors examined (i) the delay in launch and (ii) the availability of new active substances. The authors find that,
…countries experienced an average decrease in launch delay of 10.9 months (p = 0.004) after joining the EU. The effects were greatest among pharmaceuticals belonging to indications that could voluntarily participate in the CP but are not required to do so. These products are often less financially attractive to manufacturers than pharmaceutical products included in the mandatory scope. The availability of new pharmaceutical products launched was not affected. We find signs that the magnitude of the country-specific effect of centralized marketing authorization on launch delay may be influenced by strategic decisions of manufacturers at the national level (e.g., parallel trade or reference pricing).
For more details, you can read the full article. here.







