The Digital Therapeutics Alliance defines a digital therapeutics (DTx) as “evidence-based therapeutic interventions driven by high-quality software programs to prevent, manage, or treat a medical disorder or disease.” A key question is what factors U.S. payers take into account when evaluating DTx and how it differs from standard pharmaceuticals. an article from Gómez Lumbreras et al. (2024) held virtual focus groups with 21 U.S. payers to find the answer. Key considerations include:
- Need for evidence. Almost all respondents (n = 19/21, 90%) indicated that they would need a clinical trial to consider product coverage. This evidence includes data on efficacy, effectiveness, and value (including a cost-effectiveness perspective).
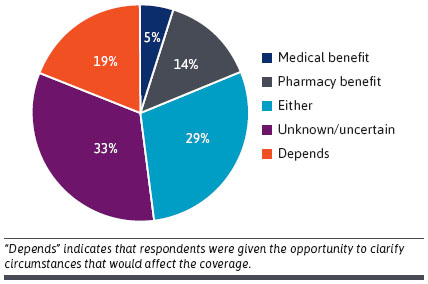
- DTx Coverage: Medical, Pharmacy or Other? Many respondents were unsure whether reimbursement should be through medical or pharmacy benefits. Most thought it would probably be the pharmacy and therapeutics committee (n = 15/21, 71%), but several participants responded “other” (n = 6/21, 29%) [see Figure below]
- FDA Regulation and Pending Legislation. Overall, 14 of 21 (66.7%) respondents would require an FDA evaluation of the DTx product to be considered for coverage (especially if covered under the pharmacy benefit). Other respondents indicated that FDA evaluation was helpful but not always necessary for coverage. Several payers cited the need for evidence beyond FDA requirements to consider coverage of a DTx product (e.g., effectiveness, value).
- Refund: NDC vs. CPT. A prescription would be required for many health plans to reimburse for a DTx product, as many policies exclude reimbursement for over-the-counter products. Participants broadly agreed that a coding system would be required and that a Current Procedural Terminology (CPT) code or a National Drug Code (NDC) would be the most efficient ways to ensure reimbursement.
- Barriers. The barriers mentioned include durability of treatment effect, cost of products, and reimbursement/payment mechanisms. Other issues included the role of DTx products in patient engagement and treatment adherence. DTxs were perceived by many to not be “authentic” treatments in part because some considered them simply “apps” and comparable versions could be downloaded online for free.
- Payer Management. Some stated that utilization management policies (e.g., prior authorization, step edits, quantity limits) could be used for DTx in the same way they are for prescription drugs. Others suggested that a DTx product could be part of a care management program rather than covered separately. Some participants explained that their organizations currently covered DTx products as part of clinical programs.
You can read the full article with useful quotes. here.








