
One approach that CMS has is Access model to cell and gene therapy (CGT). Under the CGT Access Model, CMS (at the federal level) will negotiate outcomes-based agreements (OBAs) with manufacturers, which will include pricing terms and outcomes that will be evaluated. States can then choose to participate in the contract terms negotiated by CMS or they can opt out and negotiate on their own. From CMS’s perspective, they hope that increased purchasing power will lead to lower prices (or, equivalently, more negotiated discounts). Manufacturers hope the CGT access model will speed access for patients and reduce transaction costs, since manufacturers would have to deal with far fewer than 50 state Medicaid agencies. For both parties, outcome-based contracts are onerous to implement; Having CMS will help states implement, monitor, reconcile, and evaluate the financial and clinical outcomes outlined in the OBAs.
What CGT is currently planned to be included in the CGT Access model and how much will they cost?
Two CGTs for the treatment of sickle cell anemia are planned to be included in the model. Both were approved in December 2023. “Casgevy” from Vertex Pharmaceuticals and CRISPR Therapeutics had a list price of $2.2 million per patient; Bluebird bio’s “Lyfgenia” was listed at $3.1 million per patient.
What types of reimbursements could State Medicaid Agencies receive under the CGT Access Model?
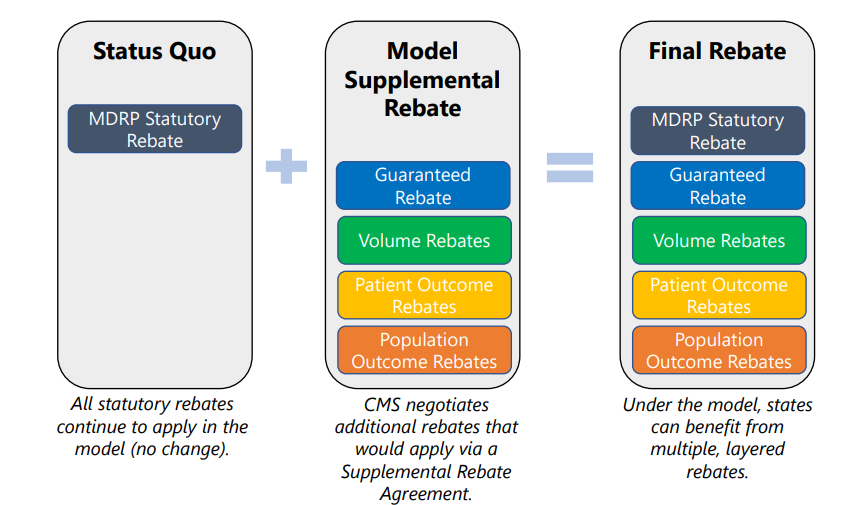
These would include not only the standard Medicaid Drug Rebate Program (MDRP) rebate (also known as Medicaid Best Price), but also additional guaranteed rebates and volume rebates, as well as rebates paid in the future based on results of patients and the population.
When would refunds be paid?
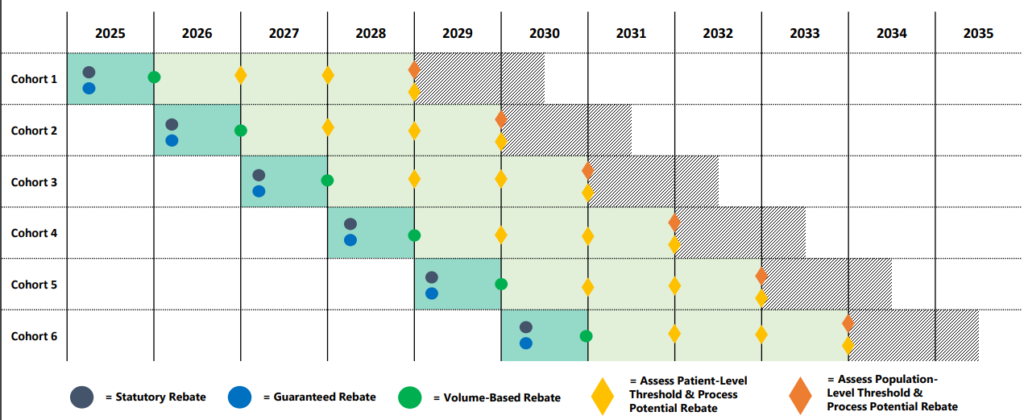
The timing of refunds depends on the type of refund. Statutory reimbursement (MDRP) and negotiated guaranteed reimbursement will be paid immediately after treatment. Volume-based discounts could come into effect at the end of the year. Finally, reimbursements for patient and population outcomes will be paid years after treatment is administered. Please note that patient outcome reimbursements may be paid annually over time to account for cases in which patients respond to therapy, but perhaps not indefinitely.
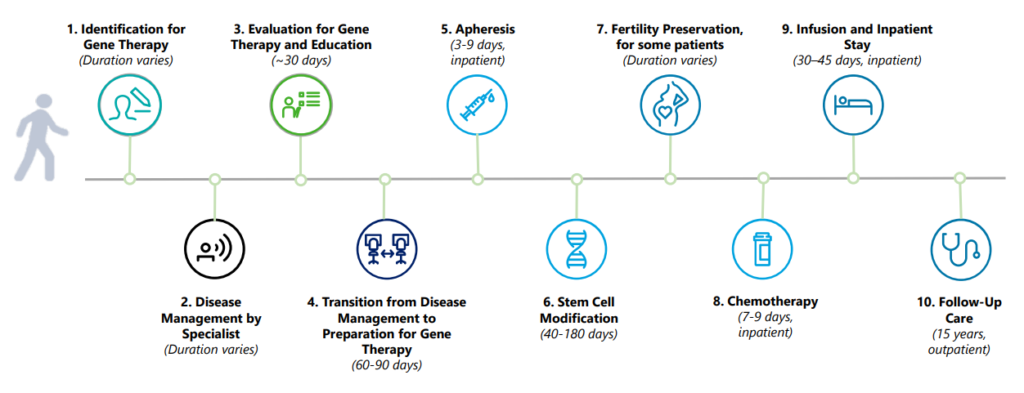
What is the process like from the patient’s perspective?
He Presentation Model of Access to the CGT: Overview for the States It has a useful timeline.
If a state decides to participate in the model, can it attempt to negotiate discounts or terms additional to those negotiated by CMS?
If a state chooses to participate in the model, it must enter into an agreement with the manufacturer that reflects the terms negotiated by CMS. Some limited variations may be permitted from state to state as necessary to comply with state legislative and regulatory requirements.
What must states do to participate in the CGT Access Model?
Requirements include:
- Execute value-based purchasing supplemental reimbursement agreements (VBP SRA) with manufacturers that reflect key terms negotiated by CMS
- Make modifications to the state plan (SPA) when appropriate
- Establish a standardized access policy for the included gene therapies.
- Remove included gene therapies from any inpatient payment package
- Require providers to follow data and claims reporting requirements.
- Ensure that beneficiaries have access to care from qualified gene therapy providers in or out of state.
- Ensure necessary transportation and related travel expenses for beneficiaries (NEMT)
- Meet T-MSIS minimum data requirements
What legislation created the CGT Access Model?
CMS states that:
The CGT Access Model was developed in response to President Biden’s Executive Order 14087, “Reduce prescription drug costs for Americans”and was first proposed in a report in response to executive order led by the Secretary of the Department of Health and Human Services.
https://www.cms.gov/priorities/innovation/innovation-models/cgt
How can I learn more?
Some useful reference material on the CMS CGT Access Model website includes:







